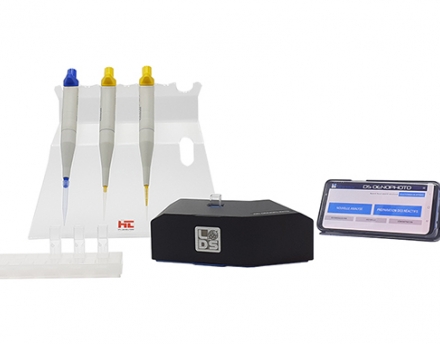
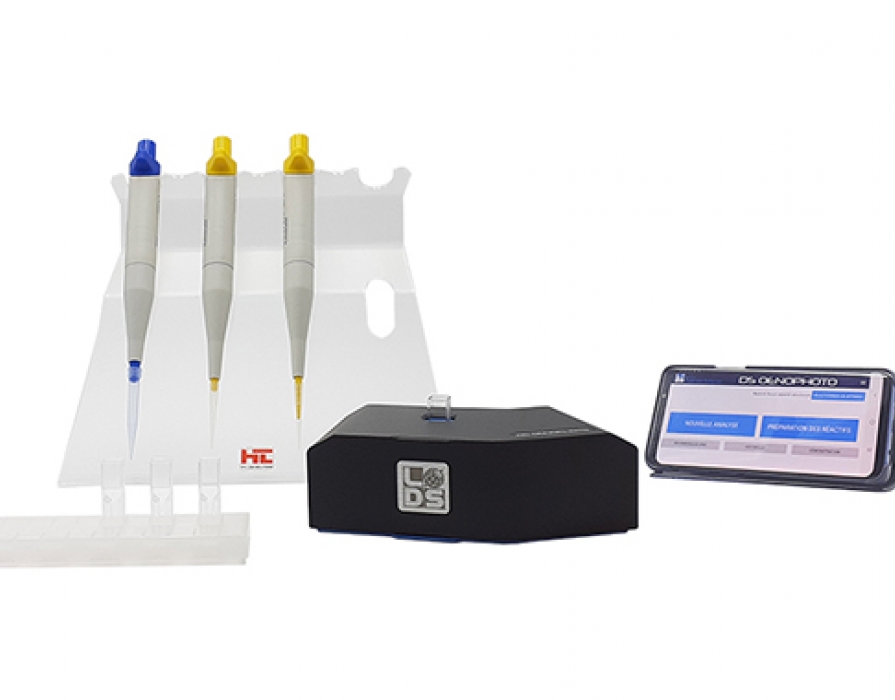
Principe
Un photomètre ou spectrophotomètre permet de mesurer l’absorbance d’une solution à une longueur d’onde précise. Une partie de la lumière qui traverse l’échantillon est absorbée par les molécules chimiques de l’échantillon, et c’est cette fraction lumineuse retenue, nommée absorbance, que l’appareil mesure. Lors de l’analyse en oenologie, on compare la réaction produite par un réactif enzymatique ou colorimétrique dans un blanc (eau), dans un standard et dans un échantillon pour pouvoir calculer la concentration du paramètre recherché dans l’échantillon. En effet, cette réaction provoque un changement de l’absorbance, proportionnel à la concentration de ce paramètre (loi de Beer-Lambert). L’absorbance de l’échantillon est alors mesurée par photométrie à une longueur d’onde déterminée.