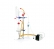
PRINCIPE
On extrait le SO2 en milieu acide par entraînement par un barbotage un courant gazeux (air ou azote) dans l’échantillon. Il est fixé et oxydé par barbotage dans une solution diluée et neutre de peroxyde d’hydrogène. L’acide sulfurique formé est dosé par une solution titrée d’hydroxyde de sodium (accessoires de titration non-fournis). Le SO2 libre est entraîné à froid, le SO2 total ou combiné est extrait à chaud.